While the chief of the paediatrics department at the Lala Lajpat Rai Hospital in Kanpur finds himself in trouble over a statement he made two days ago about 14 children with Thalassemia being detected with HIV (human immunodeficiency virus) and hepatitis infections when they presented themselves at the government hospital for blood transfusion, the issue has raised questions about the efficiency and efficacy about blood donation, testing and transfusion protocols in the country.
The chief of the paediatrics department at the government hospital, located 90 kilometres from the state capital Lucknow, had expressed deep concern at what had transpired.
A news report quoted the paediatrician expressing concern and saying it exposed the chinks in the mechanisms of blood donation and reception at health centres. These children had received infected blood from various hospitals, Arun Kumar Arya, the head of the paediatrics department was quoted as saying in the news report.
However, at a press conference in Kanpur today, October 25, the health department categorically denied that any child had come infected with HIV.
Sanjay Kala, the principal of Ganesh Shankar Vidyarthi Memorial Medical College, under which the hospital operates, rejected the report. He said the hospital has not reported HIV, Hepatitis B or Hepatitis C infections in any Thalassemia patient since 2019.
“We have ordered an investigation against Dr Arun Kumar Arya for making incorrect statements,” Kala said.
In the wake of the rumours, former chief minister Akhilesh Yadav criticised the government for its alleged inefficiency.
“It is an extremely serious issue. The government should investigate these infections and take strictest action against those responsible for it,” Yadav posted on X [formerly Twitter].
उप्र में संक्रमित ख़ून चढ़ाने से 14 बच्चों को HIV और हेपेटाइटिस का संक्रमण होना बेहद गंभीर बात है। इस लापरवाही की तत्काल जाँच हो और इस तरह की घातक गलती की सख़्त से सख़्त सज़ा दी जाए।
उप्र में चिकित्सा व्यवस्था देखनेवाला कोई नहीं है।
— Akhilesh Yadav (@yadavakhilesh) October 25, 2023
The incident has however got people talking about the loopholes and shortcomings in blood donation, reception and transfusion systems prevalent in the country.
This is not the first time such an incident has come to light in the country.
According to a news report published on February 11 this year, Maharashtra State AIDS Control Society had stated that in the last 10 years, 1,442 people have contracted HIV infections in the state after blood transfusion.
A number of times, blood donors have been found infected too. According to a news report published in June last year, 11 blood donors were found infected by HIV, 72 had hepatitis C and 54 had hepatitis B in Meerut district, Uttar Pradesh between January, 2022 and April, 2022.
Another report published in 2018 found that close to two per cent of blood donors in India were found to be infected during the testing of their blood.
Understanding the ‘window period’
Particularly worrying is the ‘window period’ where the presence of HIV and Hepatitis infections in the donated blood escape detection. The window period refers to the time it takes for the immune system to make antibodies after contracting an infection. It can range from anywhere between 18 days and 90 days for antigen/ antibody test (see figure below).
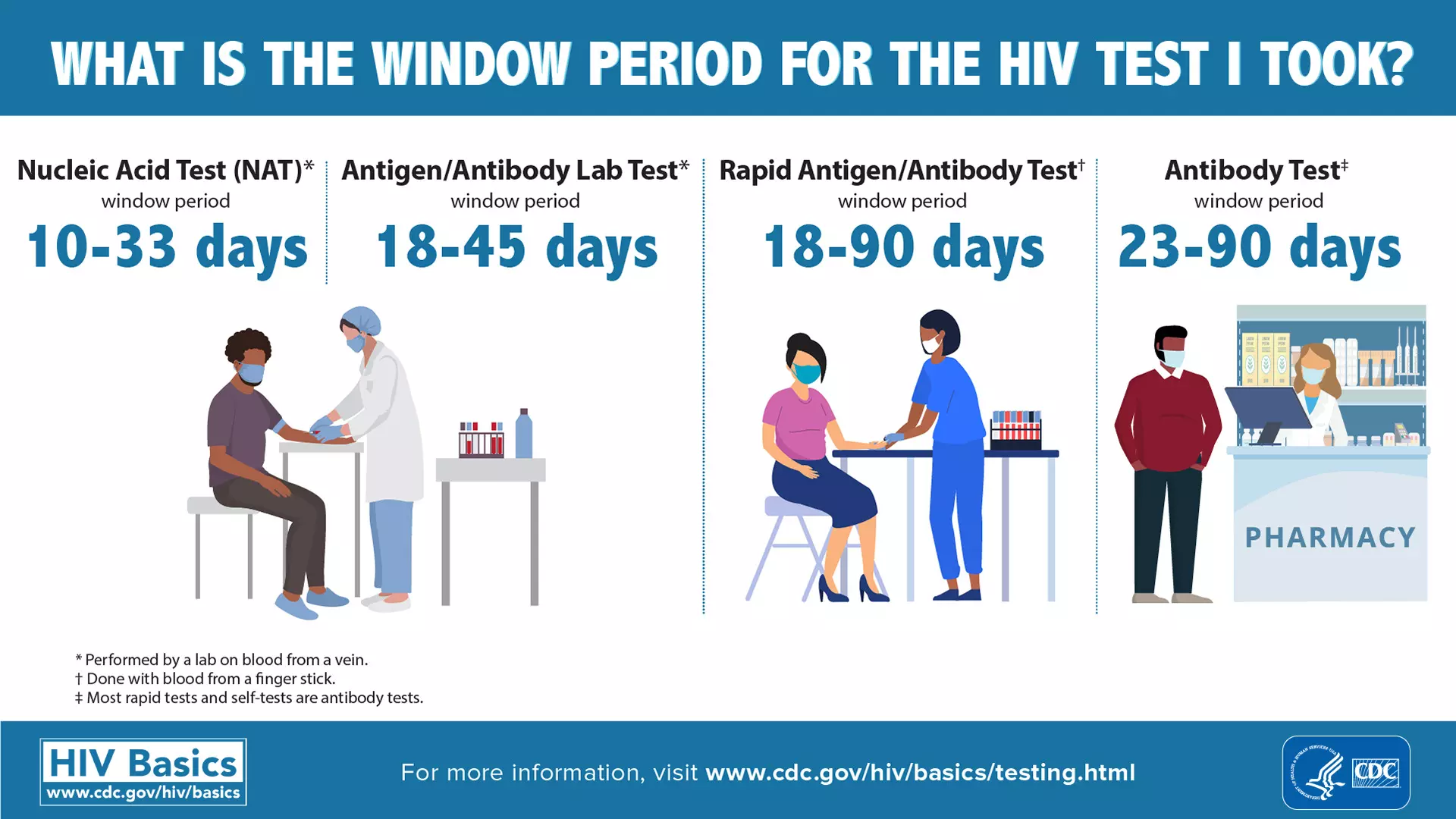
If the donor has been infected recently, then the infections can evade detection during the blood tests, and the blood could be approved for transfusion.
However, experts point out that this could be prevented if the Nucleic Acid Test (NAT) was made compulsory. Such a test can reduce the window period to 10-33 days.
“An infection will not be detected by the usual tests if it is recent. I believe a NAT should compulsorily be conducted to test blood which is taken for transfusion. The medical community has been demanding more stringent guidelines when it comes to testing blood for infection at the time of transfusion,” Harish Warbhe, director of the Nagpur-based Lifeline Blood Bank & Laboratory told Gaon Connection.
The Health Ministry’s mandatory test only screens the blood for antigens and antibodies, which are expected to check for HIV, hepatitis infections. But it isn’t accurate.
“NAT looks for genes of the viruses which makes it far more accurate than the antigen-antibody test. However, this test is expensive [costs around Rs 1,500] as a result of which many health centres don’t do it. But NAT must be made compulsory as it reduces the window period to a great extent,” Warbhe explained.
In a news report published on June 10, 2022, citing a study they have been conducting from 2013, Pune-based Jankalyan Chain of Blood Banks and Medical Services was quoted that they were able to save 198 lives by using NAT technology to identify contaminated blood from 66 units.
“In India, blood screening for HBV (Hepatitis B), HIV and HCV (Hepatitis C) by serological tests is mandatory. Sero-nonreactive blood donations are still at risk of transmitting TTIs [transfusion-transmissible infections] and hence, there is a need for an additional layer of safety with a higher sensitivity. At blood banks where NAT has been implemented, it has been observed that there is a significant reduction in the residual risk of TTI ,” Atul Kulkarni Kulkarni, director of the chain of blood banks was quoted.
The Health Ministry’s mandatory test only screens the blood for antigens and antibodies, which are expected to check for HIV, hepatitis infections. But it isn’t accurate.
To be, or NAT to be
The medical fraternity in India has been calling for a policy-intervention that makes NAT compulsory for blood transfusion.
A 2016 research study titled, Nucleic acid testing: Is it the only answer for safe Blood in India?, states that with the implementation of NAT in other countries around the world, there is a growing pressure on the transfusion services in India to adopt NAT testing. According to the research study, conducted by Indian Red Cross Society, the country has about 2,545 licensed blood centres.
“The Transfusion Services in India are fragmented, poorly regulated and the quality standards are poorly implemented. Blood Centres are still dependent on replacement/family donors and in most places laboratory testing for Transfusion transmitted infections is not quality assured, laboratory equipment is not calibrated and maintained, and validation of results is not carried out. Against the current scenario introducing NAT for screening of blood donors in India would pose a challenge,” reads the 2016 study.
A number of private hospitals in urban India have already implemented NAT testing. But in hospitals and centres run by charitable trusts and even the government, only enzyme-linked immunosorbent assay (ELISA) or rapid tests are carried out that are less effective, the 2016 pointed out.
This was reconfirmed by Wharbe of Lifeline Blood Bank & Laboratory. The prime medical institutes like AIIMS, the PGIs and the privately owned blood banks have the facilities to conduct NAT but it is not so in the rural areas,” he said.
There is the challenge of the NAT test being a strain on the resources. The 2016 study pointed out how due to poor blood collection at some centres, it was not feasible to carry out the NAT testing at all centres. There was a need to identify regional/ zonal blood testing centres first.
Implementing NAT means building of specially designed and equipped laboratories which in turn needed considerable infrastructure, equipment and manpower.
‘Blood donation vital for patients, healthy for donors’
The World Health Organization (WHO) decrees that providing safe and adequate blood should be an integral part of every country’s national healthcare policy and infrastructure.
WHO recommends that all activities related to blood collection, testing, processing, storage and distribution be coordinated at the national level through effective organisation and integrated blood supply networks.
At present, India has a digital portal called e-RaktKosh which was launched in 2016. It provides the interface for Blood Banks data management and integration. All blood banks have been advised to register on e-RaktKosh web-portal. However, the blood banks are not integrated nationally yet.
WHO says, the national blood system should be governed by national blood policy and legislative framework to promote uniform implementation of standards and consistency in the quality and safety of blood and blood products.
With inputs from Pratyaksh Srivastava, Lucknow (UP).




















